amykp got me looking to answer the question of why Hope Biosciences was not using intracranial deliver for their stem cell trial for PD. I found this:
Stem Cells: Innovative Therapeutic Options for Neurodegenerative Diseases? 2021 ncbi.nlm.nih.gov/pmc/articl...
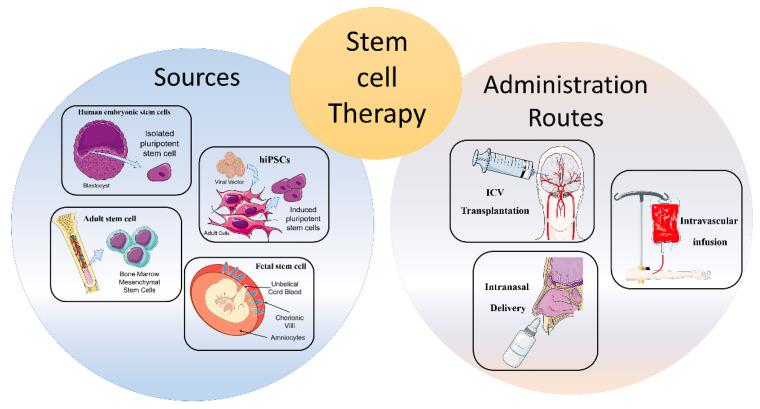
"3. Routes of Administration
Along with stem cell sources, administration routes can influence migration, distribution, and the amount of transplanted cells in the target area [28]. In addition, in accordance with different pathological conditions, the dosage of stem cells, as well as the timing of cell delivery, must be carefully considered [29]. To date, there are few studies directly comparing the efficacy of diverse transplantation routes; therefore, the optimum delivery route for specific cell types has not been determined [30].
3.1. Intracerebral or Intracerebroventricular Transplantation
Intracerebroventricular (ICV) of stem cells appeared for years to be the most precise delivery route for neural stem cells (NSC) implantation [30]. However, it showed different adverse reactions, such as motor exacerbations, syncope, seizures, and tumorigenicity. Therefore, the direct stereotaxic injection has been used less frequently in the first clinical trials [31,32].
3.2. Intravascular Infusion
Intravascular infusion, both intravenous and intra-arterial, represents a valid, safe, and less invasive alternative route of administration compared to ICV [33]. The peculiarity of this way of infusion is ability of exogenous cells to migrate towards the damaged tissue by passing the BBB [34]. Among the two routes of infusion, the intravenous administration seems less advantageous compared to the intra-arterial one, probably because of a trapping mechanism on the liver and lung area. Indeed, several studies reported a faster and more widespread cell distribution related to intra-arterial administration, since peripheral filter organs are overcome, thus leading to higher concentrations of exogenous stem cells in the target area [28,35,36,37]. However, these methods also present some disadvantages, including the standout thromboembolism microvascular occlusion, and injury exacerbation [38].
3.3. Intranasal Delivery
To date, the intranasal delivery route of administration represents a promising strategy to circumvent the BBB and to deliver drugs straight to the brain. This non-invasive way reduces the likelihood of adverse events compared to the intravascular one. Several studies have shown that stem/progenitor cells, administered intranasally, migrate to the brain through the nasal cavity and lead to positive findings in PD [39], malignant gliomas [40], and stroke [41,42,43]. In particular, transplanted cells migrate through the olfactory bulb, reach the cerebrospinal fluid (CSF) and, subsequently, the injured region. It has been demonstrated that chemokine gradients obtained from damaged cells can facilitate stem cell homing in target areas [44]. Conversely, disadvantages related to intranasal administration involve enzymatic degradation, low pH of the nasal epithelium, and individual variability that can lead to a lower release and efficacy in CNS [45]. Notwithstanding the limited studies obtained conducted so far, this route of administration may represent a potential avenue for the implantation of stem cells in the brain, specifically for neurological disorders."