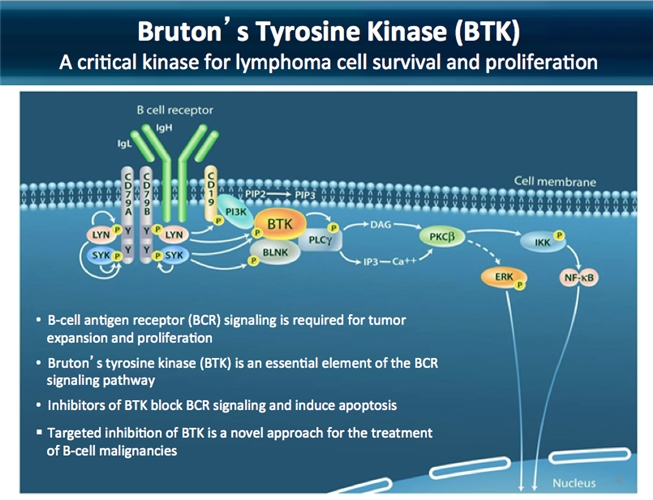
A new BTK inhibitor enters the fray in Chronic Lymphocytic Leukaemia and Small Lymphocytic Lymphoma.
ONO-4059 is currently being developed in a Phase I clinical trial for the treatment of B-cell malignancies.
A Phase I Study in CLL patients of ONO-4059
UK: Cardiff (Wales), Leicester & Plymouth (England)
France: Lille, Lyon & Montpelier
This is an open-label, multi-centre, phase I dose-escalation study to investigate the safety and tolerability of ONO-4059 given as monotherapy to patients with relapsed/refractory NHL and CLL. ONO-4059 will be administered orally, once daily(QD)as a flat dose, for up to maximum 12 cycles of treatment.
UKCTG: goo.gl/yo5dz4
Clinicaltrials.gov: goo.gl/zCkZU8
The UK trials landscape is fast changing as many novel targeted therapies enter UK trials:
UK Trials Gateway: goo.gl/vNg3Bj
UK NCRN CLL clinical trials map: goo.gl/OJPlJ8