🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨
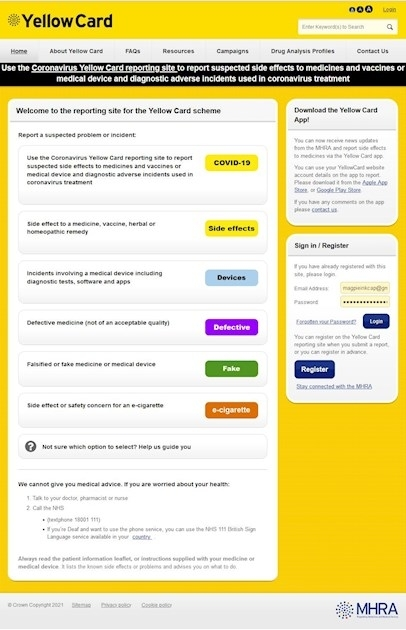
In the UK, the Medicines and Healthcare products Regulatory Agency (MHRA) operates the Yellow Card scheme for reporting issues with medicines.
They have slightly different approaches to:
🟡 Side Effects
🟡 Defective medicines
🟡 Counterfeit or fake medicines
All can be accessed by following this link:
It vitally important that we report issues. Although we might wish that we could rely on medical staff (doctors, nurses, pharmacists, etc.) to make reports, the truth is they often do not. Often no-one has told them of the issues they are having. Even if you know they have made a report, your report will add to the information. You will explain it differently and might include different details.
I do not like the culture of blaming patients which seems to be far too common. But in this case, we can only blame ourselves if we do not file reports.
If in doubt, make a report. The MHRA must expect that some reports are inappropriate and will have mechanisms in place for handling them. It isn't your responsibility to make that decision. All that can reasonably be expected is that you make reports in good faith, taking care to be as accurate as you can.
Making a report is straightforward. Depending on the issue, you might be asked to photograph the medicine (or device) and upload them. Probably a good idea to take a set of photographs before you start the report.
If there is a problem then reporting it as soon as possible might mean fewer people are affected by it. For example, if it is serious, the MHRA might initiate a recall which would prevent thousands receiving the problem product. Treat reports as URGENT!
Remember:
If you change makes and find the new one better, you might wish to report the previous make.
Retain all packaging (for example, card outer, blister packaging, Patient Information Leaflet) - even if you do not have any of the product left.
If you use some sort of daily pill dispenser, you might consider keeping any packaging for a while so it is available should you need it.
🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨
🏴 There is a page for those in Wales:
Yellow Card reporting for patients, carers and the public
On this page you will find information about what to report, how to report and who to contact for help when completing a report.
awttc.nhs.wales/medicines-o...
🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨
🏴 Mae tudalen ar gyfer rhai yng Nghymru.
Adrodd Cerdyn Melyn ar gyfer cleifion, gofalwyr a'r cyhoedd
Ar y dudalen hon fe welwch wybodaeth am beth i’w adrodd, sut i adrodd a gyda phwy i gysylltu i gael cymorth wrth wneud adroddiad.
cttcg.gig.cymru/optimeiddio...
🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨
Yellow Card Mobile App: You can now receive news updates from the MHRA and report side effects to medicines via the Yellow Card app. Please download it from the Apple App Store or Google Play Store. Key features of the app include the ability to create a ‘watch list’ of medications for which you can receive news and alerts from the MHRA. You can view numbers of Yellow Cards reported to the MHRA for medicines of interest and see previous Yellow Cards you have submitted through the app. At the moment account details are not synchronised between this website and the app, so you need to create separate accounts.
🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨
COVID-19 Issues
There is a special Covid-19 page which includes vaccines, diagnostic testing kits, a few specific medicines and several other things.
Coronavirus Yellow Card reporting site
coronavirus-yellowcard.mhra...
🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨
Outside the UK
There are other routes outside the UK. For example the USA's FDA has a reporting mechanism.
How to Report Product Problems and Complaints to the FDA
fda.gov/consumers/consumer-...
The European Medicines Agency (EMA) handles reports for centrally licensed products - each EU country handles its own for nationally licensed products.
🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨
The MHRA make statistics on Yellow Cards available on the IDAP part of the Yellow Card site.
They allow us to see numbers of reports and the issues identified.
Have a look at any medicines you take.
🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨🟨
[This is a re-post to increase visibility.]
If you want to link to this post on HealthUnlocked, copy everything below the line of equals signs and paste into your post or reply. The extra characters will add formatting.
=========
🟨 Making Yellow Card Reports 🟨