Recent comprehensive paper on the use of liquid biopsies for diagnosis and treatment of PCa. Comprehensive summary tables are provided for both purposes.
From the paper:
Introduction
The prostate-specific antigen (PSA) test is one of the most widely used screening tests for men at risk for prostate cancer (PCa).1 Recently, several advisory groups recommended against using PSA tests because of unsubstantiated outcomes. PSA tests have high false positive rates, reducing their benefit relative to the implications of unnecessary follow-ups.2 PCa-related mortality is not decreasing as fast as it should. Indeed, over the past few decades, PCa mortality among African American patients who are obese significantly worsened.3,4,5 Other lines of investigation are needed to provide a better knowledge of prostate malignancies. Many PSA-positive men develop asymptomatic PCa. Most of these tumors do not progress or they develop slowly, averting the need for active medical intervention.6 Nevertheless, invasive, painful biopsies are routinely recommended in the absence of other biomarkers. Gleason scoring (GS) by pathologists based on microscopic biopsy aids clinical prognosis. Screening depends on highly trained pathologists yet is ultimately subjective. Clinical evaluation could be more cost effective and objective. Conversely, among PCa diagnoses, approximately a third of the GS of PCa biopsies are inaccurate and underestimate the score, followed by metastasis within 5 years, which challenges clinical diagnosis and threatens patients’ lives. Therefore, discovering more accurate and focused biomarkers for PCa grading is crucial and timely. Recently developed tests may differentiate aggressive cancers from their indolent counterparts. Developing and commercializing an objective diagnostic and prognostic method for PCa detection and evaluation in a non-invasive manner could significantly benefit men.
. . .
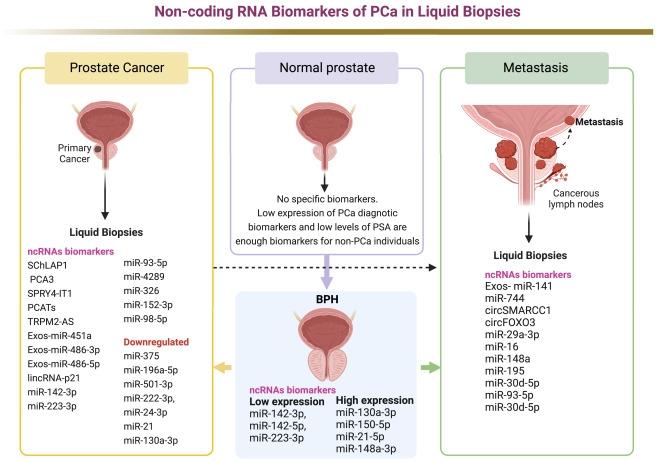
Concluding remarks
Recently, there has been a resurgence of interest in the clinical use of LBs as a minimally invasive way to diagnose and monitor PCa. Relying on solid biopsies in PCa has serious drawbacks. Tracking molecular and metabolomic biomarkers in PCa is a viable way to reduce unnecessary tissue biopsies and improve early detection of PCa. LB can also monitor the response to anticancer medicines, which may lead to better treatment options. LB shows significant efficacy in discriminating PCa from BPH and other non-PCa disorders, as presented in Figure 2. In particular, several studies have highlighted the value of UE analysis for PCa diagnosis and predicting progression and aggressiveness. UE-ncRNAs have performed essential non-invasive diagnostic tasks in patients with PCa and predicted responses to anticancer therapy. Numerous studies have emphasized the intriguing role of ctDNA/ctRNA biomarkers in the peripheral blood and urine of patients with PCa. ctDNA and ctRNA biomarkers in LBs (serum, plasma, and urine) from patients with PCa played an important role in evaluating the response to chemotherapy and numerous anticancer medications. Further, studies of metabolomic patterns in LBs from patients with PCa suggest clinical uses for distinguishing PCa from BPH disorders. Metabolic variations in PCa urine samples, while a source of diagnostic indicators, play only a modest role in distinguishing patients with PCa from patients with BPH. On the other hand, the metabolic profiling of plasma and serum biopsies can identify PCa from BPH and other non-PCa disorders. Plasma and serum studies have also identified crucial biomarkers for PCa diagnosis and monitoring medication responses. Overall, LB is effective in diagnosing PCa, primarily through early identification of cancer-related alterations in urine, plasma, and serum samples.
* * *
Full Paper can be found here:
Current advances of liquid biopsies in prostate cancer: Molecular biomarkers, Cell/Molecular Therapy Oncolytics, Review, Vol 30, P27-38, September 21, 2023 (Published:July 18, 2023)
cell.com/molecular-therapy-...
If our treatment centers adopted a wider use of these new tools, we could advance the efficacy of both PCa dignostics and treatment. Let's keep pushing them to do so.
Saluti - CaptnMojoe