3 months before I got diagnosed with PCa in May of 2013 I had been feeling tired with no libido. I will bypass my health care profession system failures as it will take up to much space.
In Feb 2012-13 I ordered several tests through Genova Diagnostics (detox genomic and complete male hormone, nutra eval, organic acid comprehensive profile). I will reference the first two with a few of so many articles wirtten on the subject of unmetabolized estrogens and their very carcinogenic DNA depurinating adducts.
Just Some of the many articles in chronological order:
A unifying mechanism in the initiation of cancer and other diseases by catechol quinones (2004)
Abstract
The first step in cancer initiation is the reaction of chemical carcinogens with DNA to form stable adducts, which remain in DNA unless removed by repair, and depurinating adducts, which detach from DNA following destabilization of the glycosyl bond. Following these results, experiments on the metabolism of estrogens, formation of depurinating DNA adducts, carcinogenicity, mutagenicity, and cellular transformation have led us to the hypothesis that certain metabolites of endogenous estrogens--in particular, estradiol(estrone)-3,4-quinones--can react with DNA to form depurinating adducts at the N-3 of Ade and the N-7 of Gua. Depurination of these adducts can generate critical mutations by error-prone repair to initiate breast, prostate, and other cancers.
pubmed.ncbi.nlm.nih.gov/156...
Potential biomarker for early risk assessment of prostate cancer (2006)
Abstract
Background: Catechol estrogen quinones (CEQ) derived from 4-hydroxyestrone (4-OHE1) and 4-hydroxyestradiol (4-OHE2) react with DNA to form depurinating--N7Gua and--N3Ade adducts. This damage leads to mutations that can initiate breast and prostate cancer. To determine whether this damage occurs in humans, urine samples from men with prostate cancer and benign urological conditions, and healthy controls were analyzed. The objective was determining whether any of the cancer patients had formed the depurinating 4-OHE1(E2)-1-N3Ade adducts.
pubmed.ncbi.nlm.nih.gov/168...
Depurinating estrogen-DNA adducts, generators of cancer initiation: their minimization leads to cancer prevention (2016)
Estrogens can initiate cancer by reacting with DNA. Specific metabolites of endogenous estrogens, the catechol estrogen-3,4-quinones, react with DNA to form depurinating estrogen-DNA adducts. Loss of these adducts leaves apurinic sites in the DNA, generating mutations that can lead to the initiation of cancer. A variety of endogenous and exogenous factors can disrupt estrogen homeostasis, which is the normal balance between estrogen activating and protective enzymes. In fact, if estrogen metabolism becomes unbalanced and generates excessive catechol estrogen 3,4-quinones, formation of depurinating estrogen-DNA adducts increases and the risk of initiating cancer is greater. The levels of depurinating estrogen-DNA adducts are high in women diagnosed with breast cancer and those at high risk for the disease. High levels of depurinating estrogen-DNA adducts before the presence of breast cancer indicates that adduct formation is a critical factor in breast cancer initiation. Women with thyroid or ovarian cancer also have high levels of estrogen-DNA adducts, as do men with prostate cancer or non-Hodgkin lymphoma.
ncbi.nlm.nih.gov/pmc/articl...
The 3,4-Quinones of Estrone and Estradiol Are the Initiators of Cancer whereas Resveratrol and N-acetylcysteine Are the Preventers (2021)
Abstract:
This article reviews evidence suggesting that a common mechanism of initiation leads to the development of many prevalent types of cancer. Endogenous estrogens, in the form of catechol estrogen-3,4-quinones, play a central role in this pathway of cancer initiation. The catechol estrogen-3,4-quinones react with specific purine bases in DNA to form depurinating estrogen-DNA adducts that generate apurinic sites. The apurinic sites can then lead to cancer-causing mutations. The process of cancer initiation has been demonstrated using results from test tube reactions, cultured mammalian cells, and human subjects. Increased amounts of estrogen-DNA adducts are found not only in people with several different types of cancer but also in women at high risk for breast cancer, indicating that the formation of adducts is on the pathway to cancer initiation. Two compounds, resveratrol, and N-acetylcysteine, are particularly good at preventing the formation of estrogen-DNA adducts in humans and are, thus, potential cancer-prevention compounds.
ncbi.nlm.nih.gov/pmc/articl...
Very Very good 2023 article on subject: I find the bolded text fascinating for monitoring our progression to castrate resistance as an early marker.
To block or not to block—hormonal signaling in the treatment of cancers
In the cell culture models of prostate cancer, it has been observed that local steroid metabolism is different in androgen-sensitive vs. Androgen-resistant cells (36). In LNCaP prostate cancer cells, as they progress toward an androgen refractory state, there are a decrease in oxidative activity and an increase in the reductive activity of 17-beta hydroxysteroid dehydrogenase 3 (17-βHSD). This results in the accumulation of bioactive estrogen (estradiol) in androgen refractory cells. Meanwhile, androgen-sensitive cells show a predominance of oxidized estrogens such as estrone (36).
Androgen-responsive LNCaP cells produce bioactive DHT and its derivatives as well as estrogen, while androgen-insensitive PC3 cells mainly produce oxidized androgen and estrogen derivatives such as androstenedione and estrone. This observation of altered steroid metabolism is crucial to the biological impact on the target cells (37).
ncbi.nlm.nih.gov/pmc/articl...
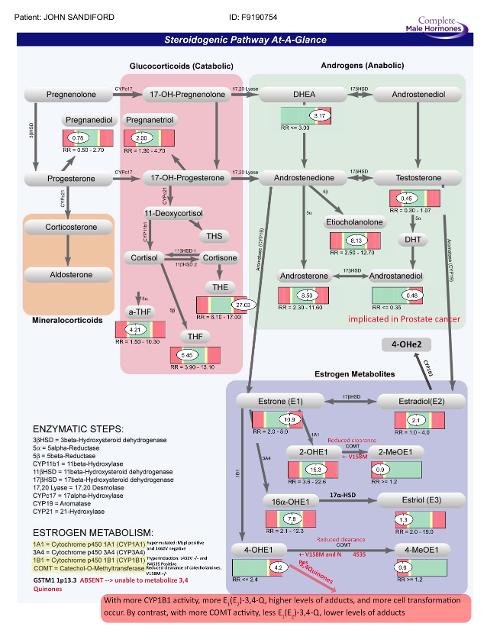
You get the picture. I could list a thousand articles on this subject, but let me show you my two Genova Diagnostic tests (above and next post) pointing directly to why my specific cyp1A1, Cyp1B1, COMT, and GST caused me to have a very unbalanced and excees 3,4 Quinones which I think is why I have no detected "cancer sequencing mutations" but have somatic MSH6-loss which does point to a germline mutation/s. (The cancer genetic sequencing does not test for CYP1A family, COMT, GSTM1