Image credit: Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes biorxiv.org/content/10.1101...
Numerous genetic mutations can cause Parkinson's[1]. The most potent among them are mutations that cause alterations in alpha synuclein. SNCA is the gene that codes for alpha synuclein. Persons with extra copies of SNCA get young onset Parkinson's at successively earlier ages depending on how many extra copies of the gene they have[2]. They produce normal alpha synuclein, but too much of it. So even a mere excess of normal a-syn can cause Parkinson's. Complete lack of alpha synuclein is not good either as demonstrated by alpha synuclein knockout mice[3].
Alpha synuclein is a protein comprised of 140 amino acids. In addition to duplication, there are half a dozen mutations that cause amino acid substitutions and result in young onset Parkinson's[2]. A mouse has been genetically modified to produce one of these, human alpha synuclein with the A53T mutation: "A53T mutant mice develop intraneuronal inclusions, mitochondrial DNA damage and degeneration, and apoptotic-like death of neocortical, brainstem, and motor neurons. " [4]. (A53T = amino acid at position 53 changed from Alanine to Threonine).
Alpha synuclein can assume an impressive variety of different shapes (conformations), either singly (monomer) or in groups (oligomer). This includes helices, ribbons (beta sheets)[5], helix of paired ribbons (fibrils), spirals (Greek key) [6]. This increases the difficulty understanding the genesis of PD. See images in my comments below for examples.
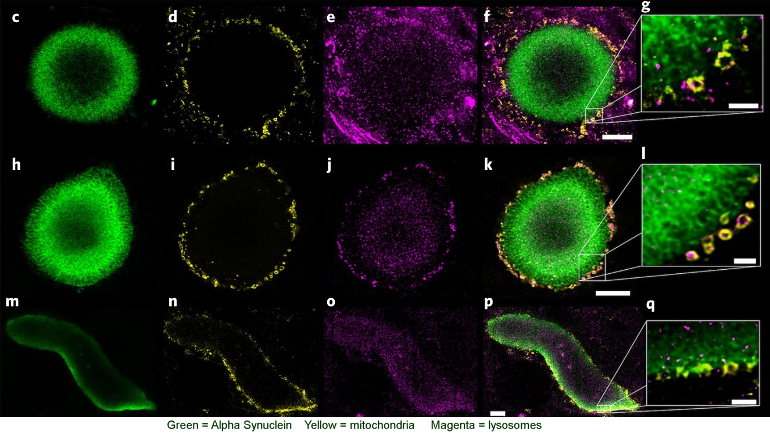
When alpha synuclein aggregates into a mass known as a Lewy body, as shown in the image at top, the result is cellular impairment and potential death. We can see that the bulk of the Lewy Body is made up of alpha-synuclein, which forms the scaffold for the structure. We can also see that the mitochondria haplessly caught up in it are in the process of being digested by lysosomes, the cell's means of recycling.
It is not only mutations that cause development of Lewy bodies. A Swiss research team recently published this very thorough work tracking the creation and development of Lewy bodies[7]: "Recently, it was shown that exogenously added preformed fibrils (PFFs) of α-syn can act as seeds to initiate the misfolding and aggregation of endogenous α-syn, in both cellular and animal models, in the absence of α-syn overexpression. .... By extending the characterization of this neuronal seeding model from day (D) 11 to D14 to D21, we were eventually able to observe the transition from fibrils to α-syn–rich inclusions that recapitulate the biochemical, morphological, and structural features of the bona fide human LBs, including the recruitment of membranous organelles and accumulation of phosphorylated and C-terminally truncated α-syn aggregates "
It takes 3 weeks from inoculation with alpha-synuclein fibrils for Lewy bodies to form. (Which is why animal studies that pre-treat with a test substance prior to applying the toxicant are not modeling Parkinson's.)
"Overall, the sequestration of proteins related to the intracellular transport inside α-syn inclusions seems to result in an impairment in the trafficking of organelles and vesicles, such as mitochondria, endosomes, lysosomes, and the synaptic vesicles, that eventually accumulate inside α-syn inclusions... Interestingly, not only proteins involved in mitochondrial transport were enriched in the insoluble fraction of the PFF-treated neurons but also proteins related to mitochondrial dynamics, the mitochondrial apoptotic pathway, and the energy metabolism pathways... This indicates that the process of inclusion formation that occurs throughout D14 to D21 might dramatically alter mitochondrial physiology, suggesting that mitochondrial dysfunction could be a major contributor to neurodegeneration in PD and synucleinopathies."
"Strikingly, our transcriptomic analysis revealed that 156 genes involved in signaling pathways related to the regulation of neuronal cell death were also significantly changed over time in PFF-treated neurons ... These genes started to be significantly dysregulated at D14. At D21, 70 genes involved in cell death signaling pathways were up-regulated in PFF-treated neurons"
α-Synuclein is emitted by neurons, both healthy and unhealthy. In the latter case it becomes a source of transmission of pathological α-Synuclein:
"In healthy and pathological settings, both monomeric and multimeric forms of α-syn are detected in the blood plasma and cerebrospinal fluid. An in vitro study overexpressing human α-syn in differentiated SH-SY5Y cells revealed that a significant amount of α-syn was detected in the culture medium as early as two hours and had accumulated over time. The extent of multimerization of α-syn at both the intracellular and the extracellular environment is also likely associated with its expression within the cells. In cells expressing low levels of α-syn, only the monomeric form of α-syn was detected in both cell extracts and the conditioned medium. Studies using H50Q and A53T mutant variants also showed a significantly enhanced secretion of α-syn into the surroundings"
To reverse disease it would seem we need to get the accumulated α-syn in the Lewy bodies to dissociate and revert to monomeric form or to find a way to induce the affected cell to eject it.
Certain toxins are potent triggers that also get this process started. The focus on mutations in this post is meant to simplify this already complex discussion as much as possible. Parkinson's is almost certainly more commonly caused by toxins than by genetic defects.
References:
1. sciencedirect.com/science/a...
2. Autosomal dominant Parkinson’s disease caused by SNCA duplications sciencedirect.com/science/a...
3. α-Synuclein knockout mice have cognitive impairments sciencedirect.com/science/a...
4. ncbi.nlm.nih.gov/pmc/articl...
5. Alpha-Synuclein Aggregation Pathway in Parkinson’s Disease:Current Status and Novel Therapeutic Approaches mdpi.com/2073-4409/11/11/1732
6. Alpha-synuclein structure and Parkinson’s disease – lessons and emerging principles link.springer.com/article/1....
7. pnas.org/doi/10.1073/pnas.1...
8. α-Synuclein at the Presynaptic Axon Terminal as a Double-Edged Sword mdpi.com/2218-273X/12/4/507
α-Synuclein preformed fibril (PFF) video: youtu.be/uz-FQrcU8XU?si=kYg...