uPSA Ultra-sensitive PSA Following Prostatectomy Reliably Identifies Patients Requiring Post-Op Radiotherapy
Using first postoperative PSA cutoff ≥0.2 ng/mL has only 46% sensitivity to detect failures. If the relapse cutoff is lowered to first postoperative uPSA ≥0.03, sensitivity substantially increased to 70% (Table 4).May 1, 2016
Any post-op PSA ≥0.03 captured all failures missed by first post-op value (100% sensitivity) with accuracy (96% specificity).
Defining failure at uPSA ≥0.03 yielded a median lead-time advantage of 18 months (mean 24 months) over the conventional PSA ≥0.2 definition.
uPSA ≥0.03 is an independent factor, identifies BCR more accurately than any traditional risk factors, and confers a significant lead-time advantage.
Biochemical failure precedes distant metastasis by about eight years
A meta-analysis quantified the success of salvage RT to decrease by 2.5% with every 0.1 PSA increment [10].
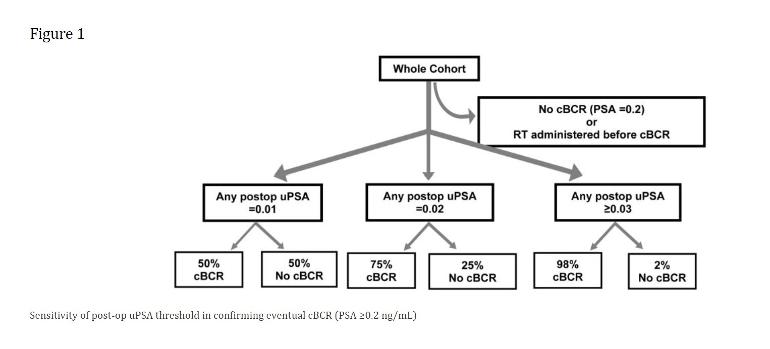
Benign uPSA patterns occurred in the range from 0.01 to 0.02 ng/mL, sometimes persisting over several repeated PSA draws.
Once the threshold was increased to uPSA ≥0.03, nearly all patients (98%) eventually relapsed (Figure 1).
Specifically, initial and continual monitoring with uPSA out to at least 5 years and at intervals not longer than 6 months between tests so that eventual failures can be identified earlier and postop RT can be initiated with a greater likelihood of success.
use of the conventional assay and cutoff (PSA ≥0.2 ng/mL) is flawed.
data showed that about half 50% of relapses missed by conventional PSA relapse criteria would be anticipated with uPSA.
The majority of our patients had uPSA failures within the first three years (82%) but a substantial number (18%) relapsed much later and therefore a closer PSA surveillance probably should extend to a minimum of 5 years.
ART (adjuvant)is better than delayed SRT (salvage), ADT (androgen deprivation) alone, or neither.