ncbi.nlm.nih.gov/pmc/articl...
Why Use Intermittent Fasting in PD?
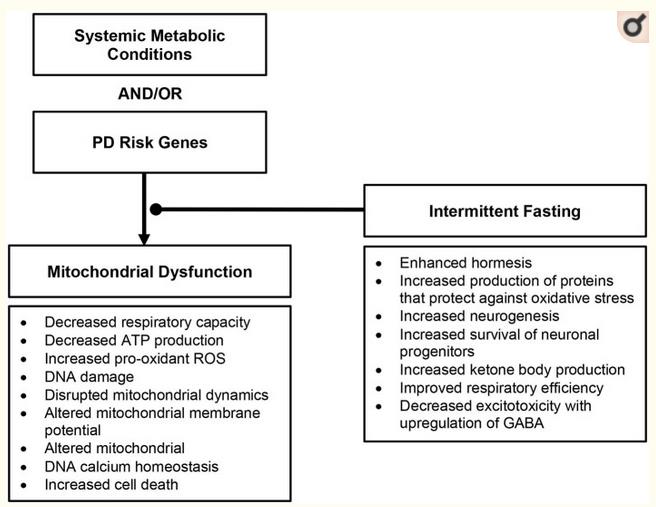
Without any available disease-modifying therapeutic options, one potential approach to slow progression of PD is to target key pathophysiologic changes in PD. Mitochondrial function is a possible target, especially in the PD cases when the mitochondria dysfunction seems to be the main and/or crucial mechanism. The benefits of IF likely originate from controlled amounts of small stress and recovery, or hormesis (38). In PD models, IF has resulted in improving insulin sensitivity (39), decreased excitotoxicity (40), reduced neurodegeneration (40), and protection against autonomic dysfunction (27, 41), and motor and cognitive decline (30). IF counteracts other pathologic features of PD by enhancing neurogenesis (37) and improving survival of neuronal progenitors (42). Moreover, the resulting ketosis may promote decreased excitotoxicity with the upregulation of GABA (43).
Importantly, IF may provide benefit for the non-motor symptoms of PD in addition to the motor symptoms. Griffioen and colleagues showed that intermittent fasting (energy restriction relative to a high energy diet) led to a decreased burden of alpha-synuclein in the brainstem that contributes to autonomic dysfunction (elevated resting heart rate, impaired cardiovascular stress response, reduced parasympathetic activity) commonly seen in PD (43). As autonomic dysfunction contributes to worse functional status, it remains an important therapeutic target in addition to motor symptoms (44, 45).
An IF dietary intervention is a more encompassing approach to mitochondrial dysfunction and its downstream consequences than nutritional supplementation and, thus, may be more beneficial to target similar pathology. IF likely influences several physiologic pathways, unlike supplementation, which only affects a narrower target (36). See Figure 3 for proposed dietary intervention.