This is a non reviewed preprint
REGEN-COV is a combination of 2 monoclonal antibodies (casirivimab and imdevimab) that bind to two different sites on the receptor binding domain of the SARS-CoV-2 spike protein. They aimed to evaluate the efficacy and safety of REGEN-COV in patients admitted to hospital with COVID-19.
Between 18 September 2020 and 22 May 2021, 9785 patients were randomly allocated to receive usual care plus REGEN-COV or usual care alone, including 3153 (32%) seronegative patients, 5272 (54%) seropositive patients and 1360 (14%) patients with unknown baseline antibody status.
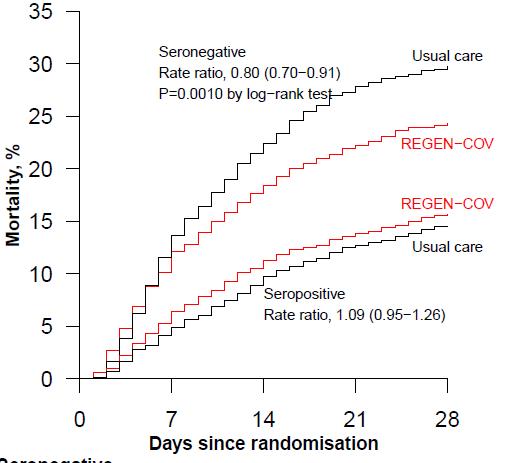
In the primary efficacy population of seronegative patients, 396 (24%) of 1633 patients allocated to REGEN-COV and 451 (30%) of 1520 patients allocated to usual care died within 28 days (rate ratio 0·80; 95% CI 0·70-0·91; p=0·0010).
In an analysis involving all randomised patients (regardless of baseline antibody status), 944 (20%) of 4839 patients allocated to REGEN-COV and 1026 (21%) of 4946 patients allocated to usual care died within 28 days (rate ratio 0·94; 95% CI 0·86-1·03; p=0·17). The proportional effect of REGEN-COV on mortality differed significantly between seropositive and seronegative patients (p value for heterogeneity = 0·001).
In patients hospitalised with COVID-19, the monoclonal antibody combination of casirivimab and imdevimab (REGEN-COV) reduced 28-day mortality among patients who were seronegative at baseline.
The trial is registered with ISRCTN (50189673) and clinicaltrials.gov (NCT04381936).
Trial details here: recoverytrial.net
Jackie