Hey, Max73. You missed one . . . and one right up your AI alley!
Salcudean and Bashashati: "In general, AI tools learn from prior examples, so there can always be a question whether the results generalized correctly. Continuous re-training and improvement as we accumulate more data will help, but integrating data and learning from various centers is a crucial step that poses challenges for AI models."
"As well, we need to understand how AI predictions work, and what are the potential pitfalls. This issue of interpretation is still an open question. It is likely that AI tools will be common in clinical use in a decade or so, to help, but not replace, the clinician."
* * *
Ali Bashashati and Septimiu Salcudean on Prostate Cancer Risk Assessment With AI - Machine learning model tested better against Gleason grading and CAPRA, by Jeff Minerd , Contributing Writer, MedPage Today, November 21, 2024.
This Reading Room is a collaboration between MedPage Today® and ASCO.
* * *
An artificial intelligence (AI) model performed better than Gleason grading and the Cancer of the Prostate Risk Assessment Post-Surgical (CAPRA-S) method when analyzing histopathology images and risk-stratifying patients with prostate cancer, researchers from the University of British Columbia reported.
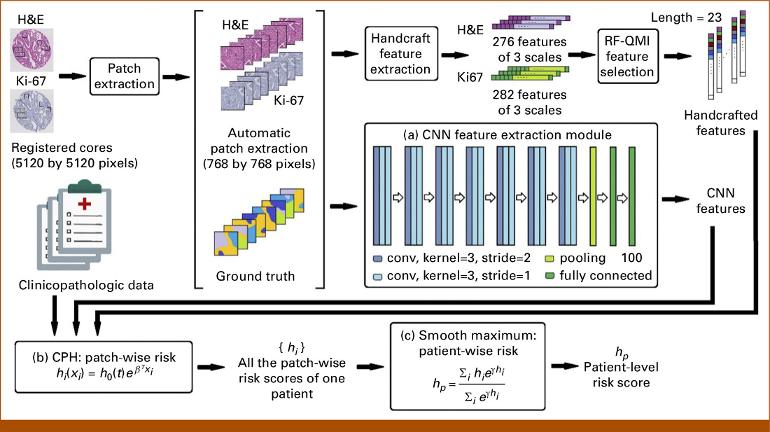
As they explained in their study in JCO Clinical Cancer Informatics, the "convolutional neural network" (CNN) model correctly reclassified as low-risk 21% of patients identified as high-risk by the CAPRA-Post Surgical model and correctly reclassified as high-risk 3.9% of patients identified as low-risk.
In the 502 radical prostatectomy patients whose tumor samples were analyzed, the team used two clinical outcomes, biochemical recurrence and overall survival, to validate their AI model.
"These findings highlight the importance of machine learning as an objective tool for histopathology image assessment and patient risk stratification," wrote Ali Bashashati, PhD, and colleagues. "With further validation on large cohorts, the digital pathology risk classification we propose may be helpful in guiding administration of adjuvant therapy including radiotherapy after radical prostatectomy."
Bashashati and co-author Septimiu Salcudean, PhD, both in the Department of Electrical and Computer Engineering, offered additional details in the following interview.
What are the limitations of Gleason scoring and the CAPRA method that make better risk-stratifying tools necessary?
Salcudean and Bashashati: Gleason grading is based on the analysis of morphological patterns and is still subject to interpretation, with significant intra-observer and inter-observer variability among pathologists, especially for low-grade cancer. This variability affects both Gleason and CAPRA-S scoring. Machine learning can provide tools for patient diagnosis and risk stratification with less subjectivity.
In the long-term, AI models can provide input on how Gleason and CAPRA-S scoring should be adjusted, or they can be directly utilized as stand-alone clinical decision-making support systems.
Your model included analysis of Ki-67-stained images in addition to the standard hematoxylin and eosin staining. Why did you add Ki-67?
Salcudean and Bashashati: Ki-67 has been shown to correlate with the proliferation of cancer cells, including prostate cancer. The dataset we had access to included this stain, providing additional information for machine learning. In a prior study, we showed that multi-stain histopathology (with Ki67 and p63) does help in prostate cancer classification.
What is a convolutional neural network? Can you explain this for people not familiar with machine learning systems?
Salcudean and Bashashati: A neural network is a functional approximation that takes an input and produces a predicted output, usually modeling a task. For example, the input could be a histopathology image, the task could be localizing the nuclei in the image, and the output the locations of the nuclei.
Internally, the neural network has several layers of neurons, each neuron having an activation function output that depends on the weighted sum of activation functions from the neurons in a previous layer. To perform a function, the weights are adjusted through "training" or "learning" for the network to match specific inputs and outputs.
In convolutional neural networks (CNN), the weights are shared so that the network performs convolutions on images. In a convolution, each pixel in the image is replaced by a weighted sum of pixels in its neighborhood; specific weights perform specific functions -- e.g., finding edges, blurring or sharpening an image.
Furthermore, pixel values of neighborhoods are aggregated or "pooled" by network layers, typically by averaging or taking the largest value. CNNs drastically reduce the number of weights needed in a model, through pooling and the use of the same learned convolution parameters regardless of the location in the image.
How did you incorporate the clinical outcomes of biochemical recurrence and overall survival into your analysis?
Salcudean and Bashashati: We used a Cox proportional hazard model that depends only on the order of events -- the patient's biochemical recurrence or death and not the exact time -- and we trained its parameters based on histopathology patches (i.e., small snapshots of the tissue) from the tissue microarray cores of the patients involved.
The AI model eventually learns relationships between the tissue images and outcome based on the data from the training cohort. At test time, the model takes as input the images and provides a risk score for a given patient.
Researchers continue to refine AI models for diagnosis and staging of prostate and other cancers. How long do you think it will be before AI tools become a standard method, and what challenges still need to be overcome?
Salcudean and Bashashati: In general, AI tools learn from prior examples, so there can always be a question whether the results generalized correctly. Continuous re-training and improvement as we accumulate more data will help, but integrating data and learning from various centers is a crucial step that poses challenges for AI models.
As well, we need to understand how AI predictions work, and what are the potential pitfalls. This issue of interpretation is still an open question. It is likely that AI tools will be common in clinical use in a decade or so, to help, but not replace, the clinician.
* * *
MedPage Article is here:
Ali Bashashati and Septimiu Salcudean on Prostate Cancer Risk Assessment With AI - Machine learning model tested better against Gleason grading and CAPRA, by Jeff Minerd , Contributing Writer, MedPage Today, November 21, 2024:
medpagetoday.com/reading-ro...
And full study is here:
Prostate Cancer Risk Stratification by Digital Histopathology and Deep Learning, Authors: Yanan Shao, PhD, Roozbeh Bazargani, MASc, Davood Karimi, PhD, Jane Wang, PhD, Ladan Fazli, MD, S. Larry Goldenberg, MD, Martin E. Gleave, MD, Peter C. Black, MD, Ali Bashashati, PhD, and Septimiu Salcudean, PhD - Publication: JCO Clinical Cancer Informatics, Volume 8, June 20, 2024:
ascopubs.org/doi/10.1200/CC...
And one to the pre-proof "prior study" by Salcudean and Bashashati mentioned in the MedPage article:
Improving Prostate Cancer Classification in H&E Tissue Micro Arrays Using Ki67 and P63 Histopathology, Yanan Shao, Guy Nir, Ladan Fazli, Larry Goldenberg, Martin Gleave, Peter Black, Jane Wang, Septimiu Salcudean, Computers in Biology and Medicine, Received Date: 26 June 2020, Revised Date: 9 October 2020, Accepted Date: 9 October 2020:
sci-hub.st/10.1016/j.compbi...
* * *
Stay S&W, Ciao - cujoe