content.iospress.com/articl...
twitter.com/ScienceofPD/sta...
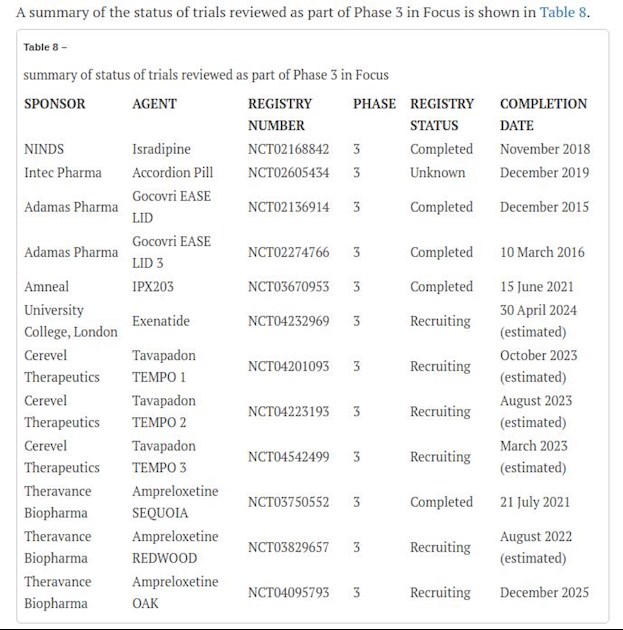
Phase 3 in focus
Since the start of the CTH section, we have covered twelve phase 3 studies in focus, ten symptomatic and two potentially disease modifying therapies. Six of the covered studies have been completed, and six continue with recruitment.
STEADY-PD III was a Phase 3, parallel group, placebo-controlled 36 months study evaluating the efficacy of isradipine 10mg daily as a disease modifying agent in early PD. The primary outcome looked at change in ON state UPDRS I-III from baseline to 36 months. The study enrolled 336 participants with 95% completion rate but failed to demonstrate slowing of disease progression [1].
Intec Pharma’s gastric retentive Accordion Pill™ Carbidopa/Levodopa (AP-CD/LD) was being evaluated in a phase 3 study for its safety and efficacy in advanced PD. Though the results have not been published, a news press release in 2019 announced that the study did not demonstrate superiority over C/L in reducing motor fluctuations in advanced PD patients [2]. Results of Gocovri EASE LID and EASE LID 3 trials have already been covered in our previous CTH issue [3]. The results were statistically significant and showed a clinically significant improvement in ON time without troublesome dyskinesia and a concomitant reduction in time with troublesome dyskinesia.
Amneal has been developing another long-acting LD/CD, IPX203 for advanced PD to reduce motor fluctuations. The results from two initial phase 2 studies of IPX203 were promising.The Phase 3, multi-center, randomized, double-blind, double-dummy, active-controlled, parallel-group study (RISE-PD) has completed with 631 participants enrolled. Results have been announced in a press release by the company but have not been published yet. According to Amneal’s website, the study successfully met its primary end point. Compared to immediate release LD/CD, IPX203 demonstrated statistically significant higher ‘Good ON’ time [0.53hr, p=0.0194) at 7 weeks. Post-hoc analysis showed increased ‘good ON’ time by 1.55 hr (p=<0.0001) per dose compared to active control by week 20. The OFF time was reduced comparatively by 0.48 hr (p=0.0252). Amneal plans to file a new drug application in mid 2022 [4].
The Tavapadon studies, TEMPO-1,TEMPO-2, and TEMPO-3 are ongoing and recruiting participants.
Exenatide is currently in recruiting phase as well.
Theravance Biopharma is evaluating ampreloxetine, a long-acting, once-daily norepinephrine reuptake inhibitor, as a potential treatment for symptomatic neurogenic orthostatic hypotension. There are three phase 3 trials designed around this molecule. SEQUOIA is a 4-week, multicentre, randomized, double-blind, placebo-controlled, parallel-group study of ampreloxetine (TD-9855) in treating symptomatic neurogenic orthostatic hypotension in subjects with dysautonomia in Multiple System Atrophy, PD, or Primary Autonomic Failure. REDWOOD is the next extension study followed by OAK, which is the open label safety and tolerability study [5]. SEQUOIA completed in June 2021 with 631 participants enrolled. Theravance biopharma announced negative results in a press release in September 2021 [6]. The study failed to meet its primary end point. Further data analysis is underway. The two sequential phase 3 trials continue to recruit but continuation is being re-evaluated.