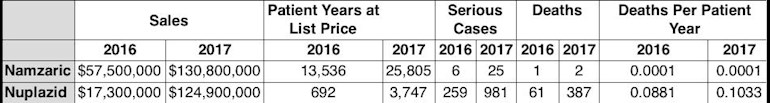
Death rate comparsion to Namzaric, for treating Alzheimer’s dementia.
" Nuplazid, when tested on people, has been a bust from the very start. The drugmaker has had a brutal time demonstrating that the medication works better than a sugar pill. For example, Nuplazid’s first clinical trial closed in March 2007, without any posting of results. The drug’s third trial ended in March 2014 but did not indicate any meaningful statistical difference between the medication and a placebo.
Statistically speaking, a drug trial whose range of results include zero is judged to be a failure in that the drug’s therapeutic benefits are deemed to be too small to be of medical consequence.
Faced with a third failure, Acadia’s management might have decided it had reached the end of the road in trying to successfully develop the drug. But due to a provision of the Food and Drug Administration Safety and Innovation Act, however, in August 2014 Acadia was able to get Nuplazid classified as a breakthrough therapy, a status conferred on therapies with “substantial treatment effects” in their initial clinical tests.
It was a curious decision, given Nuplazid’s track record and the FDA’s plainly stated requirement for a breakthrough therapy to have “substantial treatment effects observed in early clinical development.”
For the FDA’s part, Dr. Mitchell Mathis, the agency’s division director of psychiatry products, told the panel reviewing Nuplazid in March 2016 that awarding the breakthrough designation hinged on the fact that no other FDA-approved drugs existed for treating Parkinson’s disease psychosis, as well as the frequency that these patients were being placed in nursing homes, which he called “a harbinger of death.”
More baffling still was the FDA’s willingness to assess whether Nuplazid worked based on “a negotiated evidentiary standard” that eliminated long-standing evaluation criteria.
In a November analysis, Quarter Watch, a publication of the Institute for Safe Medication Practices, flagged several ways the approval process of Nuplazid was unusual. For example, the FDA permitted the drug’s efficacy to be measured against an index of nine psychotic symptoms — as opposed to the standard 20-point scale — and the patients in the study were exclusively advanced cases (the most likely to be responsive to any drug). The agency also allowed Acadia to stage only a single trial (rather than the usual two) and to run it just in North America, where its previous results had been marginally stronger."
...
The physician responsible for leading the FDA’s medical review of Nuplazid, Dr. Paul Andreason, recommended against the medication’s approval, asserting there was an “unacceptably increased, drug-related, safety risk of mortality and serious morbidity.” Andreason worked for 26 years for the U.S. Public Health Service until leaving it in 2016; he spent 13 years with the FDA. His no vote was unusual in that it publicly revealed fault lines inside the division over what constitutes an appropriate level of patient risk.
“The FDA’s division of psychiatry usually reviews drugs with an eye towards a lifetime of use,” said Andreason, in an interview with the Southern Investigative Reporting Foundation. “We rarely saw high morbidity in something under our consideration; [Nuplazid] is the first drug where a psychiatrist has to understand life is at risk.”
Andreason’s presentation to the FDA committee, summarizing the research he conducted and outlined in the meeting’s transcript, described Nuplazid’s results as presenting a “safety signal.” Specifically, 49 of the 459 patients who took the drug on a long-term basis either died during the trial or within 30 days of its completion. This represents a fatality rate that’s higher than 10.6 percent. For the placebo group, according to the FDA’s briefing document, the figure was one out of 210 in a key trial. For the 901 patients who took at least one dose of Nuplazid, the associated fatality rate was slightly higher than 5.4 percent. Andreason also observed that the patients taking Nuplazid had about a two-and-a-half-fold increase in the observed risk ratio — defined as reported incidents of an infection, a patient’s deterioration in mental clarity or death — when compared with the rate for those taking the placebo."