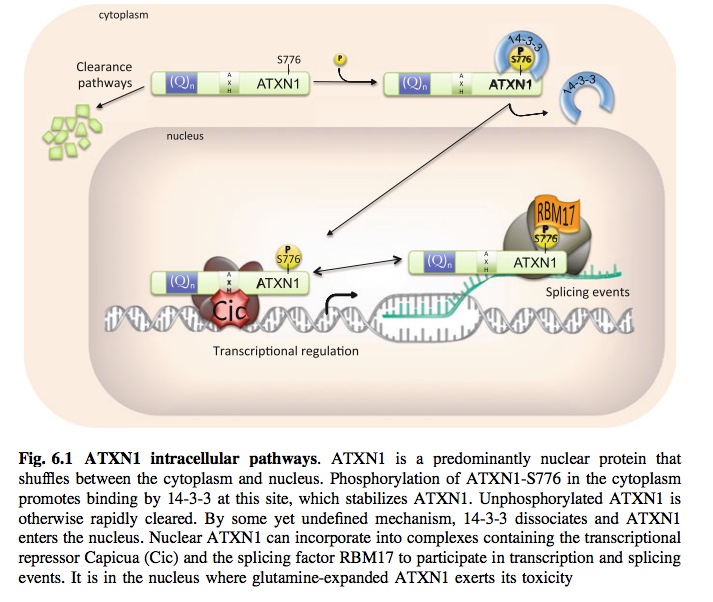
So in my never ending quest to keep abreast of the research on cures for SCA1, I came across several articles and a patent application that are most intriguing. As most folks know, many of the SCAs share a common element in that the body produces a mutant protein that is toxic to cells in the brain, and that is what causes the disease. For many years research was focused on ways to repair the genetic defect, but those methods required surgery of the brain or direct injections into the brain and they presented numerous hurdles to science. The research is ongoing in these areas. In the last decade though researchers have begun to realize that maybe just clearing the protein might be enough to stop the disease. This post is about one group of molecules that scientists are examining. (For those who don't already know who I am or have not followed any of my posts I urge you to click on my name after reading this post to learn about other molecules that have been shown in cell cultures and mouse models to help clean out mutant proteins). Anyways, back to the main point here . . .
-
-
It appears that researchers learned years ago that a class of drugs used in cancer treatments that "inhibited" cancer cells from reproducing could also "inhibit" the bad protein in patients with SCA1 from becoming toxic. The paper linked below was from 2014 and is entitled:
-
The design and delivery of a PKA inhibitory polypeptide to treat SCA1
-
onlinelibrary.wiley.com/doi...
-
I got to that article because I came across a newly published paper:
-
Reduction of protein kinase A-mediated phosphorylation of ATXN1-S776 in Purkinje cells delays onset of Ataxia in a SCA1 mouse model
ncbi.nlm.nih.gov/pubmed/297...
-
and, what I thought most interesting is that the names on the second paper are all super big-wigs in the world of SCA including Harry Orr and Huda Zoghbi.
-
but, what really got my interest is when I learned that the authors of the first paper from 2014 have applied for a patent on a molecule and method for treating SCA1 and other polyQ diseases:
patents.google.com/patent/U...
-
In the end though, this only makes me further infuriated at researchers and drug companies because here you have a line of research that has the potential to be a true cure, and what's more there are many other Protein Kinase Inhibitors ALREADY on the market like, Tasigna (nilotinib), that may be beneficial. In fact the second paper to which I linked above screened over 50 potential drug candidates and found several that were effective in mice, BUT nobody in the US or Europe seems to be in any hurry to try this for ataxia in humans even though the drugs are already being used in humans for other diseases. . . . AAHHHHHHHHHHH!!!
-
Ah well, c'est la vie . . .